Science Chemistry Chemistry questions and answers Draw the major organic product of the Bronsted acid-base reaction. Include all lone pairs and charges as appropriate. Ignore any counterions. :0: NaOH Select to Draw Using the arrows provided in the image, predict the product of this reaction. Include all lone pairs. Ignore inorganic byproducts.
Solved Draw the major organic product of the Bronsted | Chegg.com
VIDEO ANSWER: Draw the reaction between the compounds. That’s a mixture of two acids. … Organic Chemistry Janice Gorzynski Smith 4th Edition. Find All Video Solutions for Your Textbook. Question. … Draw the major organic product of the Bronsted acid-base reaction between the compounds shown below. Include all lone pairs and charges as

Source Image: youtube.com
Download Image
Book: Organic Chemistry I (Walker) 6: Acids, Bases, and Electron Flow
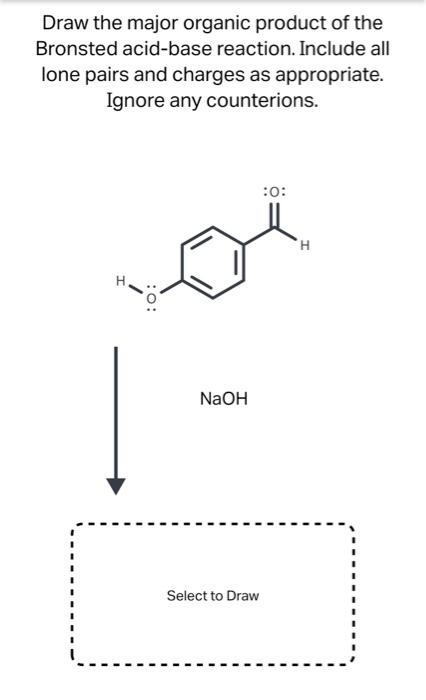
Source Image: chegg.com
Download Image
Solved Draw the organic product of the Bronsted acid-base | Chegg.com An acidbase reaction, according to the Brønsted-Lowry definition, is a transfer of a proton from one molecule or ion to another. When ammonia is dissolved in water, it undergoes the following reversible reaction. NH3 (aq) base + H2O(l) acid ⇌ NH+4 (aq) acid + OH− (aq) base NH 3 ( a q) + H 2 O ( l) ⇌ NH 4 + ( a q) + OH − ( a q) base

Source Image: scribd.com
Download Image
Draw The Major Organic Product Of The Bronsted Acid-Base Reaction
An acidbase reaction, according to the Brønsted-Lowry definition, is a transfer of a proton from one molecule or ion to another. When ammonia is dissolved in water, it undergoes the following reversible reaction. NH3 (aq) base + H2O(l) acid ⇌ NH+4 (aq) acid + OH− (aq) base NH 3 ( a q) + H 2 O ( l) ⇌ NH 4 + ( a q) + OH − ( a q) base We have already discussed in the previous chapter one of the most familiar examples of a Brønsted-Lowry acid-base reaction, between hydrochloric acid and hydroxide ion: In this reaction, a proton is transferred from HCl (the acid, or proton donor) to hydroxide (the base, or proton acceptor ).
Organic Chemistry Klein 2nd Edition Test Bank | PDF | Acid | Chemical Equilibrium
Science Chemistry Chemistry questions and answers Draw the major organic product of the Bronsted acid-base reaction between the compounds shown below. Include all lone pairs and charges as appropriate. Ignore any counterions. (๑) 1 eq. base Draw or tap a new bond to see suggestions. Draw the major organic product of the Bronsted acid-base reaction. Draw the major organic product of the Bronsted acid-base reaction. Include all lone pairs and char… – YouTube

Source Image: youtube.com
Download Image
85 Conjugate Base Royalty-Free Images, Stock Photos & Pictures | Shutterstock Science Chemistry Chemistry questions and answers Draw the major organic product of the Bronsted acid-base reaction between the compounds shown below. Include all lone pairs and charges as appropriate. Ignore any counterions. (๑) 1 eq. base Draw or tap a new bond to see suggestions. Draw the major organic product of the Bronsted acid-base reaction.

Source Image: shutterstock.com
Download Image
Solved Draw the major organic product of the Bronsted | Chegg.com Science Chemistry Chemistry questions and answers Draw the major organic product of the Bronsted acid-base reaction. Include all lone pairs and charges as appropriate. Ignore any counterions. :0: NaOH Select to Draw Using the arrows provided in the image, predict the product of this reaction. Include all lone pairs. Ignore inorganic byproducts.
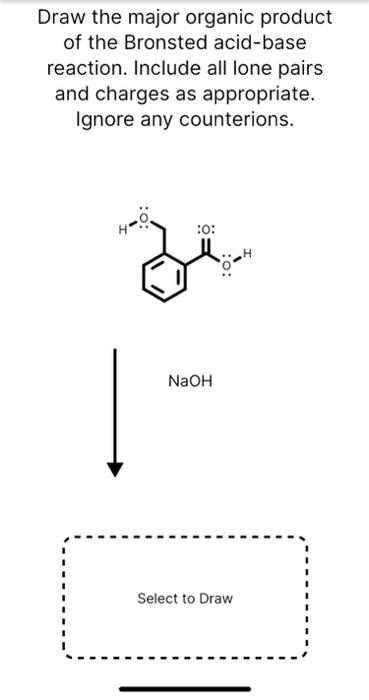
Source Image: chegg.com
Download Image
Solved Draw the organic product of the Bronsted acid-base | Chegg.com Book: Organic Chemistry I (Walker) 6: Acids, Bases, and Electron Flow
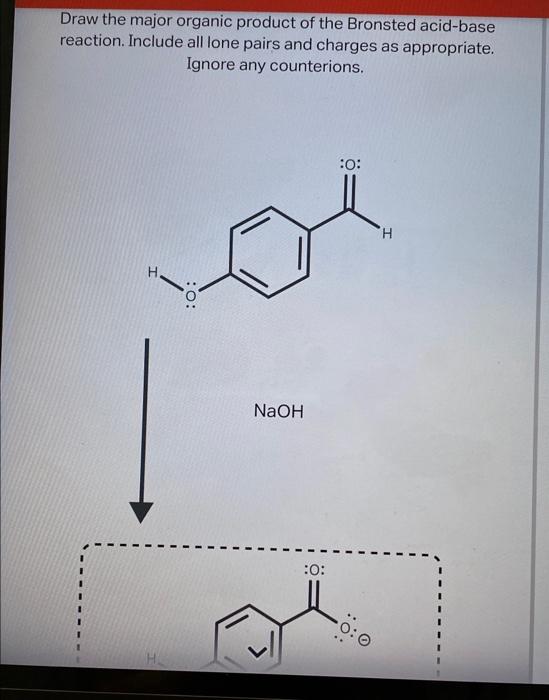
Source Image: chegg.com
Download Image
85 Conjugate Base Royalty-Free Images, Stock Photos & Pictures | Shutterstock The lower the pKa of an acid, the stronger or weaker the acid. draw the major organic product of the Bronsted acid-base reaction. Include all lone pairs and charges as appropriate. Ignore any counterions. Propoxide (CH3CH2CH2O- ) is a larger molecule than ethoxide (CH3CH2O- ), yet they are equally basic. Explain why they are equally basic.

Source Image: shutterstock.com
Download Image
Solved] Draw the organic product of the Bronsted acid-base reaction…. | Course Hero An acidbase reaction, according to the Brønsted-Lowry definition, is a transfer of a proton from one molecule or ion to another. When ammonia is dissolved in water, it undergoes the following reversible reaction. NH3 (aq) base + H2O(l) acid ⇌ NH+4 (aq) acid + OH− (aq) base NH 3 ( a q) + H 2 O ( l) ⇌ NH 4 + ( a q) + OH − ( a q) base
Source Image: coursehero.com
Download Image
Compound Bisphenol A | PDF We have already discussed in the previous chapter one of the most familiar examples of a Brønsted-Lowry acid-base reaction, between hydrochloric acid and hydroxide ion: In this reaction, a proton is transferred from HCl (the acid, or proton donor) to hydroxide (the base, or proton acceptor ).

Source Image: slideshare.net
Download Image
85 Conjugate Base Royalty-Free Images, Stock Photos & Pictures | Shutterstock
Compound Bisphenol A | PDF VIDEO ANSWER: Draw the reaction between the compounds. That’s a mixture of two acids. … Organic Chemistry Janice Gorzynski Smith 4th Edition. Find All Video Solutions for Your Textbook. Question. … Draw the major organic product of the Bronsted acid-base reaction between the compounds shown below. Include all lone pairs and charges as
Solved Draw the organic product of the Bronsted acid-base | Chegg.com Solved] Draw the organic product of the Bronsted acid-base reaction…. | Course Hero The lower the pKa of an acid, the stronger or weaker the acid. draw the major organic product of the Bronsted acid-base reaction. Include all lone pairs and charges as appropriate. Ignore any counterions. Propoxide (CH3CH2CH2O- ) is a larger molecule than ethoxide (CH3CH2O- ), yet they are equally basic. Explain why they are equally basic.